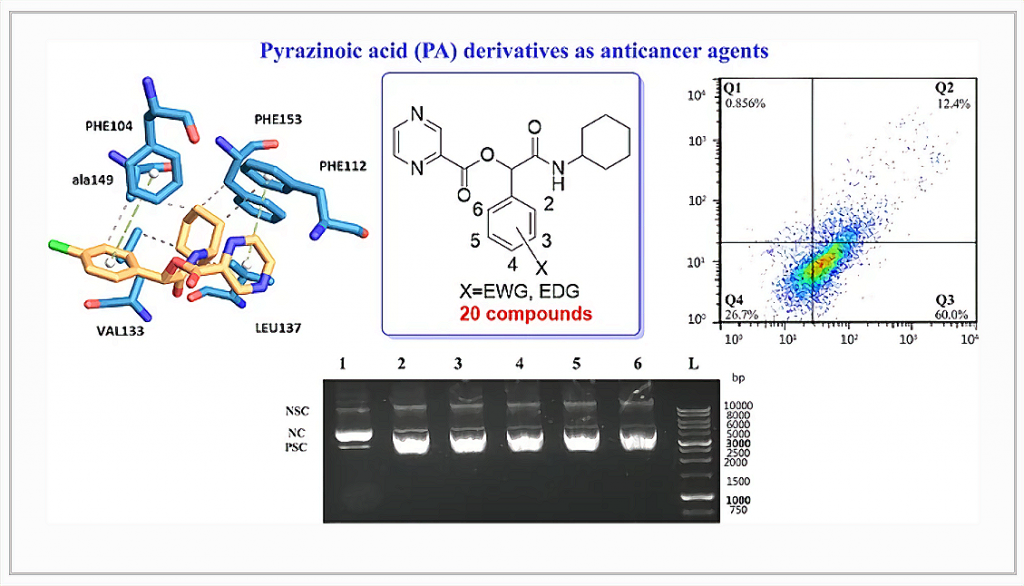
Pyrazinoic acid or pyrazine-2-carboxylic acid (PA), due to its nitrogenous heteroaromatic ring, can be explored as an anticancer agent. Here, a series of twenty novels PA derivatives have been synthesized and characterized using IR, NMR, and mass spectrums. Their cytotoxic activity was evaluated against three different cancer cell lines, including lung (A549), breast (MCF-7), and colon (HT-29). P16, the most potent compound, showed moderate cytotoxicity with IC50 of 6.11, 10.64, and 14.92 μM, against the A549, MCF-7, and HT-29 cell lines, respectively. Furthermore, the effect of this compound against MRC5 as a non-tumoral lung cell line, exhibited a selectivity index of 9.02. The apoptotic induction activity of P16 was also performed on the A549 cell line. The results showed that as the concentration of the compound increases (from 3 to 6 μM), the percentage of induction of apoptosis increases from 8.54% to 72.4%. Electrophoretic gel mobility shift assays showed that P16 was able to reactive oxygen species (ROS) induce DNA cleavage in the presents of H2O2 (1.0 mM) in high doses. Molecular docking was also applied to anticipate the binding locations and the binding of the synthesized compound with Bcl-2 apoptosis regulator and DNA as their proposed targets.