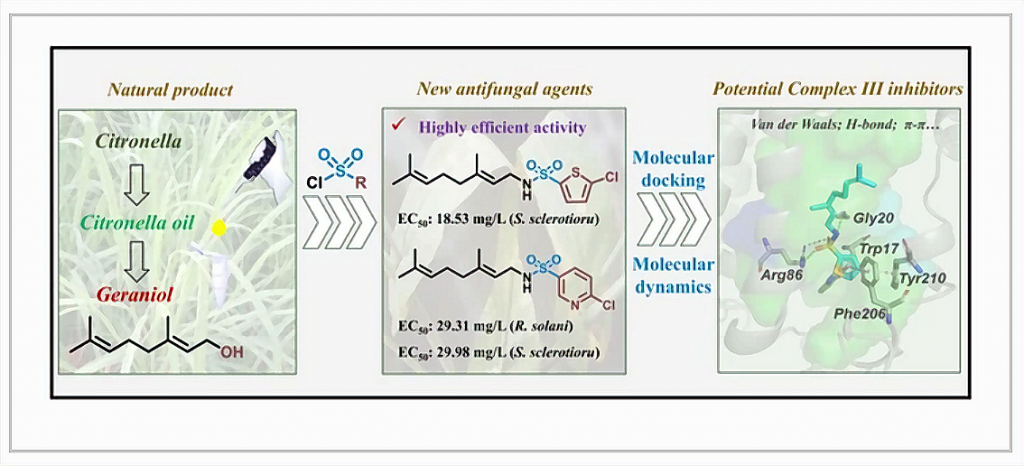
Essential oils (EOs), as unique natural products, are promising resources for the discovery of green agrochemicals. The main ingredient geraniol of citronella oil was found to exhibit substantial antifungal activities in this study. Therefore, a series of novel geranyl aromatic sulfonamide compounds were synthesized and found to display considerable antifungal activities. Two geranyl thiofuran-sulfonamide compounds 4c-1 (median effective concentration (EC50) against Rhizoctonia solani: 24.97 mg/L and EC50 against Sclerotinia sclerotiorum: 27.26 mg/L), 4c-2 (EC50 against S. sclerotiorum: 18.53 mg/L) and one geranyl pyridine-sulfonamide compound 4d-2 (EC50 against R. solani: 29.31 mg/L and EC50 against S. sclerotiorum: 29.98 mg/L) were screened as “star molecules” due to their excellent antifungal activities. The preliminary structure-activity relationship (SAR) study revealed that the introduction of various aromatic heterocycles maybe an efficient protocol to improve the fungicidal activities of geranyl aromatic sulfonamide compounds. The molecular mechanisms of the geranyl aromatic sulfonamide compounds were clarified by performing molecular docking and molecular dynamics (MD) simulations. Three “star molecules” of these geranyl aromatic sulfonamide compounds were found to bind to Complex III through several hydrogen bonds and π-interactions with crucial residues TRP17, GLY20 etc. Their binding free energies were calculated to be strong ranging from −50.60 to −39.44 kcal/mol by MM/GBSA method, which suggested the geranyl aromatic sulfonamide compounds were potential Complex III inhibitors. The main component originating from the natural plant EOs ought to be studied in the future to discover novel pathogenic fungicidal candidates