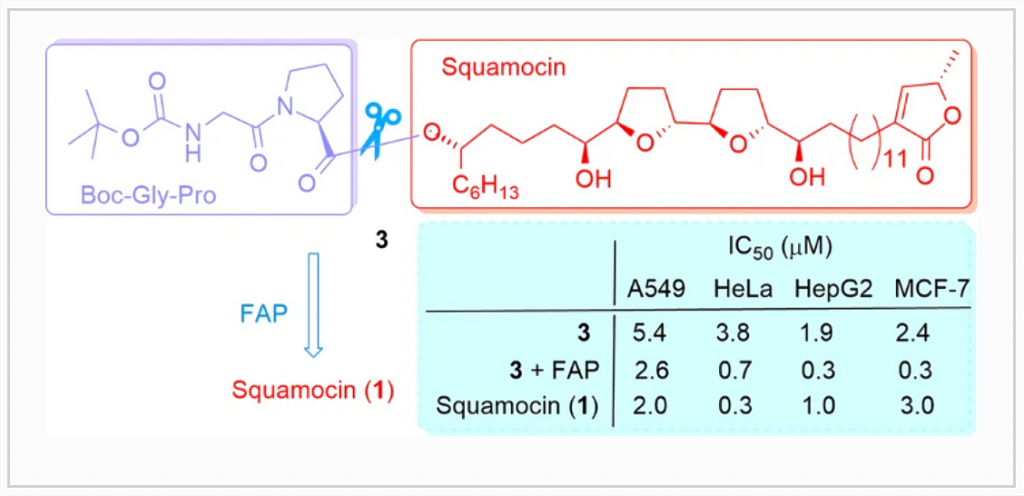
Annonaceous acetogenins are potent ubiquinone-linked NADH oxidase inhibitors and have potent anticancer activity. Their usage in the clinic is limited because of their significant toxicity to normal cells. To obtain novel low toxicity antitumoral prodrugs, squamocin and bullatacin were covalently linked to N-butoxycarbonyl protected glycine-proline dipeptide (Boc-Gly-Pro), which may be recognized and cleaved by fibroblast activation protein (FAP), a serine protease overexpressed on the surface of tumor-associated fibroblasts. Ten squamocin and bullatacin derivatives were synthesized by attaching Boc-Gly-Pro either directly or through 6-aminocaproic acid linker to a hydroxyl group of squamocin or bullatacin. All derivatives showed high potency to inhibit 4T1 breast cancer cell line growth in the sub-µM to µM range. Compound 8 was the most active (IC50 0.30 μM) and displayed higher activity than squamocin. Most derivatives, however, display reduced potency by up to 50 folds compared to the parent drug. In the presence of FAP enzyme, the anticancer potency of compound 3 against A549, HeLa, HepG2 and MCF-7 cells was increased by up to eight folds. The data suggest that Boc-Gly-Pro-acetogenin prodrugs may show improved therapeutic potential of these acetogenins by reducing the drug doses and the toxic side effects.